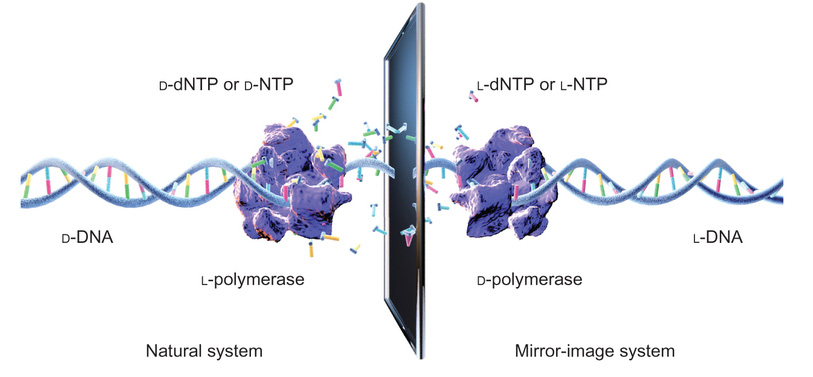
The building blocks of life are strangely asymmetrical. Amino acids can come in one of two kinds – left or right – that are mirror images of one another. Synthesise them chemically, and you get half of each. But for obscure reasons, living things make their proteins out of just left-handed variety. Meanwhile “natural” DNA only comes in one form – the double helix twists in a right-handed manner (D-DNA), and never to the left (L-DNA). Left-handed proteins, right-handed DNA.
But that doesn’t stop synthetic biologists from trying to tinker around with the chemicals of life. What if you could get right-handed proteins to make left-handed DNA? Well, it seems a group from Tsinghua University, Beijing have done just that (paywall, sorry). Zimou Wang et al. chemically synthesised a small DNA polymerase from a virus, using D-amino acids, and showed it could copy a short stretch of L-DNA. The reactions were painfully slow by normal standards – it seems the viral polymerase is very inefficient compared to the ones we use in the lab for PCR – but sure enough, it worked. It seems that, as expected, there is no chemical or physical reason why life should use one side hand over another. It just happens to be that way.
Who knows – if there is life elsewhere in the universe, it could be just like staring into the looking glass.
